Harland Medical Expands Leadership in Coating Testing with the Launch of the CTS1100™ Coating Thickness Testing System
Harland is the industry standard in measuring lubricity and durability with their FTS friction testing systems. The innovation continues with the introduction of the CTS1100 coating thickness testing system, a first of its kind machine designed for accurately and easily measuring coating thickness on medical devices.
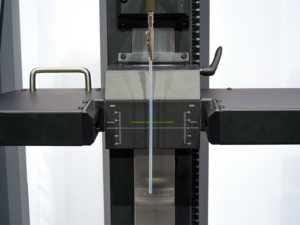
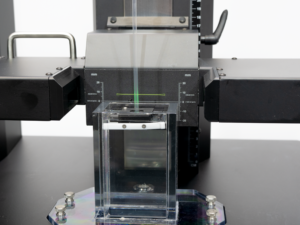
MINNEAPOLIS, MINN., April 12, 2022— Harland Medical Systems, Inc., a global medical device coatings leader, today announced the release of the CTS1100 Coating Thickness Testing System. A first of its kind testing system, the CTS1100 allows medical device manufacturers to accurately and easily measure coating thickness on their devices.
Because low-friction, hydrophilic coatings swell when hydrated, coating thickness and its effect on overall part diameter is a critical variable in the design of today’s catheters, introducers, delivery systems and guidewires. The CTS1100, using a precision LED micrometer, is specifically designed to measure the diameter of a part before coating, dry and hydrated to accurately provide a total picture of the effect of the coating on part diameter.
Harland’s proprietary TestingWorks™ software, used in the CTS1100, accounts for slight variations in the position of a device sample, providing more accurate measurements. It’s able to measure, record and display part diameter via spot check, programmed locations or via continuous scan over the coated part length, up to 60 cm. For quick and repeatable measurement, the software allows users to program testing protocols and output customizable reports.
“Innovation has always been at the heart of what we do at Harland,” said Jon Anderson, chief executive officer, Harland Medical. “As medical devices become more complex they require tighter tolerances and clearances. We saw the need and developed a testing system specifically for medical devices that will give device manufacturers an accurate measurement of how coating effects part diameter.”
For self-administered catheters, dryout time is a critical measurement. The CTS1100 is able to perform an automated dryout test making tedious testing easy. Using a device’s critical dryout diameter, the CTS1100’s real-time precision micrometer can continuously measure the diameter of a sample, automatically determining the dryout time.
If a device manufacturer is not ready to invest in capital equipment, Harland offers testing services as part of their Coatings 360™ tailored approach. As the industry’s only comprehensive source for high-performance coatings, equipment and production coating services— Harland has everything needed to coat a medical device on time and within budget.
Harland will be launching the CTS1100 at MD&M West, Booth 2248 in the Anaheim Convention Center, from April 12-14. For more information about Harland’s CTS1100, please visit harlandmedical.com/products-and-services/testing-equipment/cts1100/.
About Harland Medical
Harland Medical was founded in 2003 and is located in Minneapolis, Minnesota – a recognized global center of healthcare excellence and innovation. Harland Medical continues this tradition with our comprehensive suite of medical coatings, equipment and services for producing successful medical device coatings on healthcare products across the world. To learn more, visit harlandmedical.com.