Lubricent® UV
PFAS Free UV Curable Hydrophilic Coatings
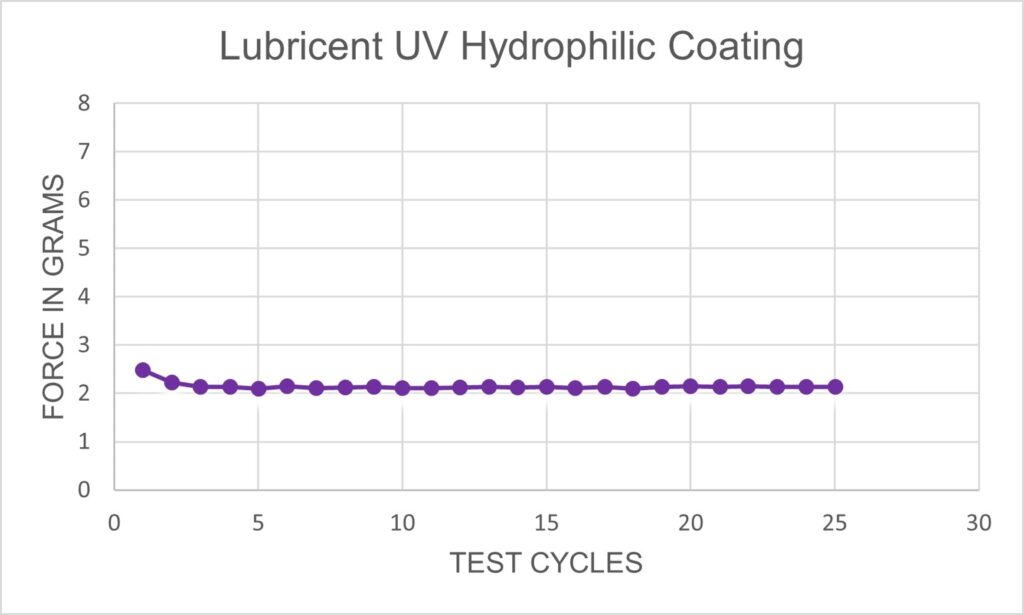
Harland’s Lubricent UV hydrophilic coatings provide exceptional friction reduction with state-of-the-art durability. Lubricent coatings enable catheters, sheaths, introducers, guide wires and other medical devices to navigate through the most difficult, tortuous vessels and anatomies. The PFAS free solution blends a hydrogel-forming polymer with a proprietary photo-reactive compound dissolved in isopropyl or ethyl alcohol. With Lubricent hydrophilic coating on your medical device, you’ll get best in class lubricity and durability no matter the application.